Parallel import of pharmaceuticals from the EU
ORIGINAL | AFFORDABLE | AUTHORIZED
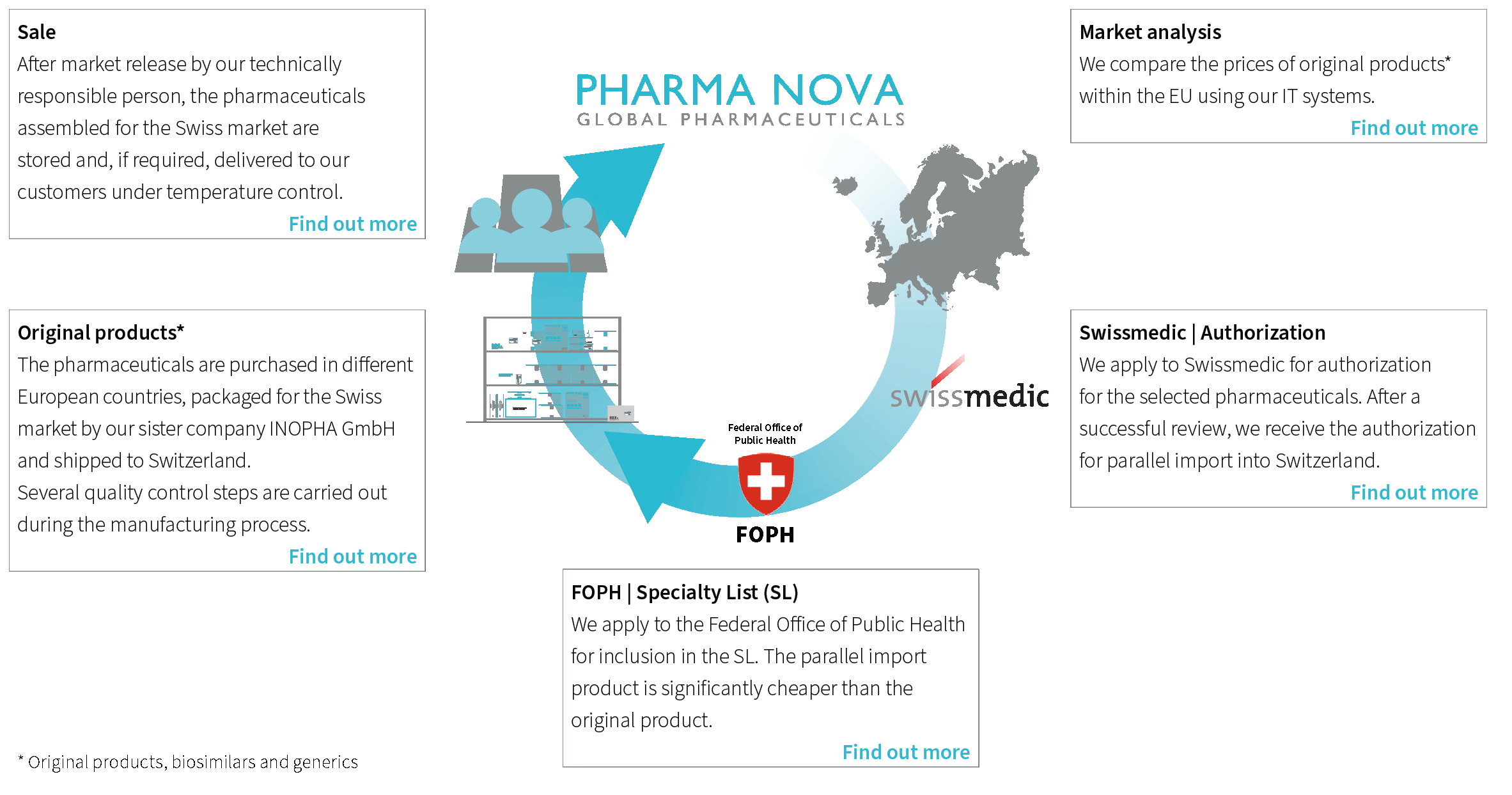
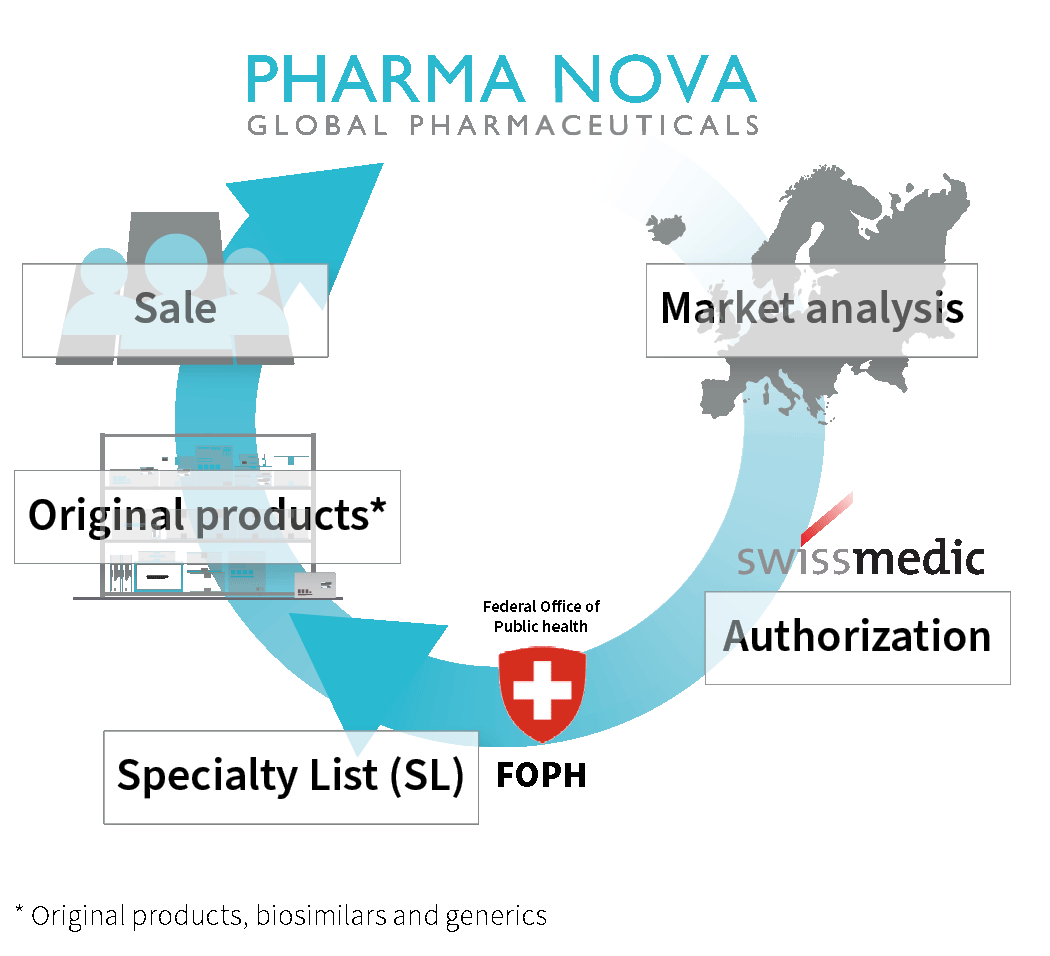
Market analysis
- We assist you in identifying the pharmaceuticals you require.
- For these pharmaceuticals, we then compare the prices and availability of the original products* within the EU using our IT systems.
Swissmedic | Authorization
- If the desired pharmaceuticals have not yet been authorized, we apply to Swissmedic for authorization. This process can take up to 12 months.
- After a successful review, we receive the authorization for parallel import into Switzerland.
FOPH | Specialty List
- We apply to the Federal Office of Public Health for inclusion in the specialty list (SL). This list contains all original products* reimbursed by compulsory health insurance as well as their parallel imports.
Original products*
- As soon as Swissmedic has issued the authorization, our sister company INOPHA GmbH purchases the pharmaceuticals from qualified European suppliers and stores them under temperature-controlled conditions once they have passed an incoming goods inspection.
- Following this, INOPHA GmbH packages the medicines for the Swiss market in accordance with Swissmedic specifications, subjecting them to several quality control steps during repackaging. All steps are always GMP (“Good Manufacturing Practice”) compliant.
- After the final inspection by the Qualified Person at INOPHA GmbH, the products are transported to the Swiss interim storage facility under temperature control.
Sale in Switzerland
- After market release for the Swiss market by our technically responsible person, the pharmaceuticals are stored in our Swiss warehouse.
- From here we deliver them to our customers.
- We always ensure GDP (“Good Distribution Practice”) compliant handling.
* Original products, biosimilars and generics